Kugadzira mamorekuru e makemikari makemikari, maatomu ezvinhu zvakasiyana-siyana kana zvinhu zvinofanirwa kusangana pamwe nenzira yakatsiga, uye izvi zvinogona kuitika nenzira dzakasiyana siyana kuburikidza nehunhu hwakaumbwa hunowanikwa neatomu yega yega, iyo, sekuziva kwedu, inoumbwa nenuclear inobhadhariswa yakakomberedzwa negore remagetsi.
Maekitironi anopomerwa zvakaipa uye anoramba ari padyo nenikosi nekuti iyo remagetsi simba inovakwezva. Iyo yepedyo elektroni iri kune iyo nucleus, inowedzera simba rinodiwa kuti riitwe kuti iburitse.
Asi hazvisi zvese zvinhu zvakafanana: vamwe vane tsika yekurasikirwa nekunze maerekitironi egore (zvinhu zvine yakaderera ionization simba), nepo vamwe vachivabata (zvinhu zvine yakakwira electron kuwirirana). Izvi zvinoitika nekuti maererano nemutemo weLee octet, kugadzikana kunoenderana nekuvapo kwemaerekitironi masere muhombodo yekunze kana orbital, kazhinji muzviitiko zvakawanda.
Zvino sei panogona kuve nekurasikirwa kana kuwana maerekitironi, maoni echaji yakatarisana anogona kuumbwa, uye kukwezva kwemagetsi pakati peyoni yechaji yakapesana kunoita kuti idzi dzibatanidze uye dzigadzire zviri nyore makemikari makemikari, umo chimwe chezvinhu chakapa maerekitironi uye chimwe chakachigamuchira. Saka kuti izvi zviitike uye a ionic chisungo Izvo zvinodikanwa kuti pane mutsauko kana delta ye electronegativity pakati pezvinhu zvinobatanidzwa kweinenge 1.7.
Iyo ionic chisungo inowanzoitika pakati pesimbi yesimbi uye isiri yesimbi: iyo atomu resimbi rinopa imwe kana akawanda maerekitironi uye zvichidaro inoumba maoni (cations) anechaji chaiyo, uye iyo isingaenzaniswi inoiwana uye inova iyo yakashata isina mhosva (anion). Iyo alkali uye alkaline pasi simbi ndizvo zvinhu zvinowanzo gadzira cations zvakanyanya, uye halogen uye okisijeni zvinowanzove anion.
Kazhinji, makemikari anoumbwa neionic zvisungo vari zvinodziya pakamuri tembiricha uye yakanyanya kusungunuka poindi, yakanyungudika mumvura. Mukugadzirisa ivo vari chaizvo makondakita akanaka emagetsisezvo iwo akasimba electrolyte. Simba reratiyiti yeiyo ionic yakasimba ndiyo inoratidza simba rinokwezva pakati peion yeiyo yakasimba.
Inogona kukushandira:
- Mienzaniso yeCoverlent Bonds
- Magnesium oxide (MgO)
- Mhangura sulphate (CuSO4)
- Potassium iodide (KI)
- Zinc hydroxide (Zn (OH) 2)
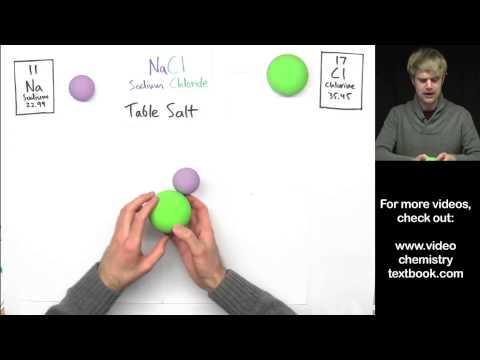
- Sodium chloride (NaCl)
- Sirivheri nitrate (AgNO3)
- Lithium fluoride (LiF)
- Magnesium chloride (MgCl2)
- Potassium hydroxide (KOH)
- Karusiyamu nitrate (Ca (NO3) 2)
- Karusiyamu phosphate (Ca3 (PO4) 2)
- Potassium dichromate (K2Cr2O7)
- Disodium phosphate (Na2HPO4)
- Iron sulphide (Fe2S3)
- Potassium bromide (KBr)
- Calcium kabhoni (CaCO3)
- Sodium hypochlorite (NaClO)
- Potasium sulphate (K2SO4)
- Manganese chloride (MnCl2)


